IonEx™ — Pure Innovation in Ion Exchange.” Removing Ions, Delivering Purity
IonEx™ — Advanced Ion Exchange Solutions by InfraCorp
InfraCorp’sIonEx™ technology represents the forefront of ion exchange innovation, delivering versatile and reliable water treatment solutions for industrial and commercial applications.
Whether softening hard water, demineralizing industrial feedwater, polishing condensate in power plants, producing ultrapure water for sensitive processes, or treating complex wastewater streams, IonEx™ provides exceptional performance and operational efficiency.
IonEx™ also plays a critical role in cutting-edge resource recovery processes, including:
- Lithium Recovery from brines and process waters, enabling sustainable battery raw material supply
- Rare Earth Elements (REE) Extraction, supporting advanced manufacturing and clean energy technologies
With a broad footprint across diverse industries—including chemical synthesis, manufacturing, food processing, mining, power generation, agriculture, lithium recovery, and REE extraction—IonEx™ adapts to your unique water treatment and resource recovery challenges with scalable, sustainable, and customized solutions.
ION EXCHANGE OPERATION CYCLES
Ions are charged atoms or molecules. When an ionic compound dissolves in water, it dissociates into cations (positively charged) and anions (negatively charged). Ion exchange (IX) processes take advantage of this property by selectively replacing ions in a solution based on their electrical charge. This is achieved by passing the solution through an ion exchange resin, which provides a fixed matrix containing reactive sites where the exchange reactions occur. The resin’s functional groups hold counter-ions that are swapped with target ions in the water, enabling selective removal or replacement according to the application.

Ion Exchange Operation Cycles
Ions are charged atoms or molecules. When an ionic compound dissolves in water, it dissociates into cations (positively charged) and anions (negatively charged). Ion exchange (IX) processes take advantage of this principle by selectively replacing ions in a solution based on their electrical charge. This is accomplished by passing the water or process stream through an ion exchange resin—a porous, polymeric matrix containing fixed ionic sites. These sites hold exchangeable counter-ions that are swapped with targeted ions in the fluid, enabling selective removal or replacement.
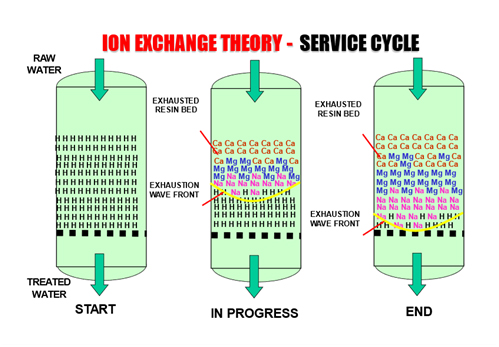
An ion exchange system operates in repeated service and regeneration cycles:
-
- Service Cycle – The feed water or solution flows through the resin bed. Target ions in the solution exchange with the counter-ions on the resin’s functional groups. Over time, the resin becomes saturated with unwanted ions, reducing its capacity.
- Backwash (Optional) – Water flows upward through the resin bed to expand and reclassify it. This removes trapped solids, redistributes resin beads, and prepares the bed for regeneration.
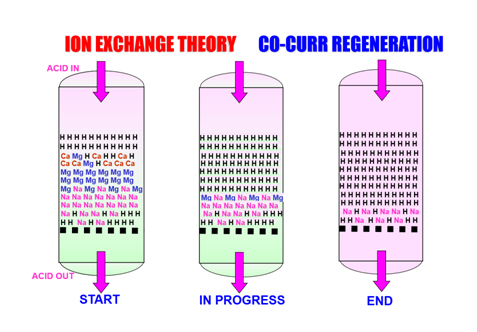
- Regeneration Cycle – A regenerant solution (e.g., sodium chloride for softening resins, hydrochloric acid or caustic soda for demineralization resins) is introduced to displace the captured ions and restore the resin to its original ionic form.
- Slow Rinse / Displacement – The regenerant is slowly flushed through the bed to ensure complete ion replacement and remove displaced ions from the resin pores.
- Fast Rinse – High flow of clean water removes residual regenerant, returning the resin to service quality.
- Return to Service – The resin is now fully regenerated and ready for the next service cycle.
This cyclical operation allows ion exchange systems to deliver continuous, high-quality water treatment for applications ranging from water softening and ultrapure water production to specialized industrial separations such as lithium recovery.
SPECIALTY RESINS
While specialty IX resins are highly effective for specific industrial applications, their greater specificity generally means greater expense and narrower adoption than conventional IX resins. Chelating resins, for example, are used extensively for concentration and removal of metals in dilute solutions, such as Cobalt (Co2+) and Mercury (Hg and Hg2+). Similarly, magnetic ion exchange (MIEX) resins are often deployed for removal of natural organic matter from feed water.
INDUSTRIAL ION EXCHANGE
Ionizable groups attached to the resin bead determine the functional capability of the resin. Industrial water treatment resins are classified into four basic categories:
- Strong Acid Cation (SAC)
- Weak Acid Cation (WAC)
- Strong Base Anion (SBA)
- Weak Base Anion (WBA)
DEMINERALIZATION (DEIONIZATION) OF WATER
Purpose:
Remove both cations and anions (all dissolved mineral ions) from water to produce demineralized (ion-free) water.
Process:
Two types of ion exchange resins are used in sequence to remove all ionic species:
- Strong Acid Cation (SAC) Resins:
- Function: Remove positively charged ions (cations) like Ca²⁺, Mg²⁺, Na⁺, etc.
- Form used: Hydrogen form (H⁺), represented as R–SO₃H
- Effect: Cations in water are replaced by hydrogen ions.
- Strong Base Anion (SBA) Resins:
- Function: Remove negatively charged ions (anions) like Cl⁻, SO₄²⁻, NO₃⁻, etc.
- Form used: Hydroxide form (OH⁻), represented as R–NH₃OH
- Effect: Anions in water are replaced by hydroxide ions.
Result:
H⁺ from cation resin and OH⁻ from anion resin combine to form pure water (H₂O), effectively removing dissolved salts and minerals
ION EXCHANGE – MIXED BED SYSTEMS
A mixed bed ion exchanger contains a homogeneous mixture of strong acid cation (SAC) resin in the hydrogen (H⁺) form and strong base anion (SBA) resin in the hydroxide (OH⁻) form within a single vessel. As water passes through the bed, cations are exchanged for hydrogen ions and anions are exchanged for hydroxide ions. The hydrogen and hydroxide ions combine to form pure water, delivering exceptionally high treated water quality.
Mixed beds are often used as a final polishing step after a primary demineralization stage (such as separate-bed cation/anion exchange or reverse osmosis) to achieve ultrapure water with conductivity as low as 0.055 μS/cm (18.2 MΩ·cm resistivity).
Key Characteristics
- High Purity Output – Removes residual ions to sub-ppb levels.
- Compact Design – Combines cation and anion exchange in one vessel, eliminating multiple units.
- Final Polishing – Ideal for semiconductor manufacturing, power plant condensate polishing, and pharmaceutical water systems.
- Sensitive to Feed Quality – Best performance is achieved when feed TDS is <100 µS/cm to minimize regeneration frequency and resin fouling.
